Fight against Covid: the Italian vaccine produced by Reithera passes phase 1. "Italy is competitively able to play important games on the ability to generate sophisticated biomedical technology". Lo said the president of the Higher Health Council Franco Locatelli at the presentation of the results of phase 1 of the trial of the Italian anti-Covid vaccine Reithera GRAd-CoV2. "We aim to develop 100 million doses of the vaccine per year". Thus the president of the Italian vaccine developer Reithera, Antonella Folgore.

Meanwhile, the Ministry of Health makes it known that today they have been registered 15.378 new positive cases (yesterday 10.800). The increase in tampons is 135.106 tampons (yesterday 77.993). The new deaths are 649 (yesterday 348), while the discharged healed are 16.023 (yesterday 16.206). Overall, the positive cases are 569.161, in practice 1.297 less than yesterday.
The Italian vaccine has passed stage 1
“We enrolled 100 people and 45 were vaccinated with different doses and all came to the end for the safety assessment. The vaccine had no serious adverse effects in the first 28 days after vaccination. A better result than Moderna and Pfizer which have had side effects. The peak of antibody production at 4 weeks remains constant and the vaccine is a single dose ". He said it il scientific director of Spallanzani Giuseppe Ippolito to the presentation of the results of phase 1 of the experimentation of Reithera's GRAd-CoV2 vaccine.
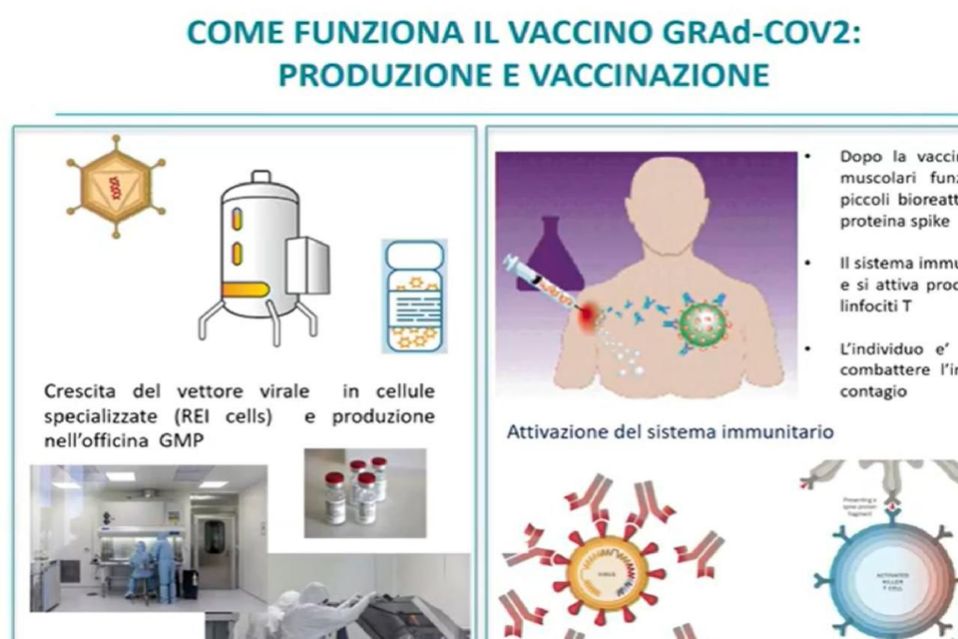
In this phase 1 the Reithera vaccine “has been shown to be safe, to have the ability to induce the immune response of adults. In fact, the response is similar to that of other two-dose vaccines. 92,5 percent of the vaccinated had detectable antibody levels and with a single dose we have results in line with Moderna and Pfizer. The levels of antibodies - stressed Ippolito - “are consistent with a single administration scheme. Now phase 2 awaits us, for which the commitment of the State is necessary ”.
Reithera Grad-Cov2, first encouraging results
"Let's try to achieve some independence also in the supply of vaccines", stressed Domenico Arcuri, extraordinary commissioner for the Covid-19 emergency. “The national executive has allocated sufficient resources to finance the subsequent development of the ReiThera trial through a public company. The government - Arcuri reiterated - will also enter Reithera with an equity operation. The development contracts will be used to finance research and incremental stabilization of production ”.

“The first results of the vaccine developed by Reithera and tested at the Spallanzani Institute are encouraging. If the data obtained so far are confirmed, we will have in the coming months an effective and safe vaccine with a single dose, instead of two doses. It is a vaccine that it will be produced entirely in our country. It is important to continue investing in Italian research and its scientific excellence ". These are the words of the Minister of Health, Roberto Speranza, at the presentation of the results of the Reithera vaccine at the Spallanzani hospital.